Reducing Energy Use in Solvent Evaporation: Smarter Solutions for DMSO and DMF Handling
In many labs, evaporating high-boiling solvents like DMSO or DMF quietly drains more energy than you think. Traditional rotary evaporators keep water baths, vacuum pumps, and chillers running nonstop—equipment that eats up power and demands constant attention.
But more innovative evaporation strategies are emerging. By rethinking your setup, you can cut power use, recover samples more consistently, and free up valuable lab time—all without compromising reliability. Some labs are already proving it’s possible to skip the bulky equipment and still get better results. Here’s how you can, too.
Table of Contents
1. Why Energy Use in Evaporation is a Hidden Problem?
2. What Makes DMSO and DMF So Challenging to Evaporate?
3. How Smart Evaporator™ Reduces Energy and Time
4. Smart Evaporator™ in Action: Trusted by Leading Institutions
1. Why Energy Use in Evaporation is a Hidden Problem
Solvent evaporation, especially for high-boiling solvents like DMSO and DMF, quietly consumes more energy than many labs expect.
Most labs use rotary evaporators with additional equipment: heating baths, vacuum pumps, and chillers. These run continuously, even during downtime. They aren’t just inefficient—they’re also easy to overlook when auditing energy use.
A standard rotavap system consumes 1.5–3.0 kWh per day. Multiply that by several units and 250 working days per year, and you’re looking at thousands of kilowatt-hours.
Labs focused on sustainability or cost-reduction need to rethink their evaporation tools. Energy efficiency isn’t just about lighting or freezers anymore. It’s in your solvent workflows too. Addressing these hidden energy demands can help improve efficiency over time.
2. What Makes DMSO and DMF So Challenging to Evaporate?
Widely Used—But Hard to Remove
DMSO (dimethyl sulfoxide) and DMF (dimethylformamide) are common solvents in pharmaceutical and biological research. Their strong solvating power makes them ideal for:
Drug synthesis
Peptide analysis
NMR sample preparation
However, these same properties make them notoriously difficult to evaporate.
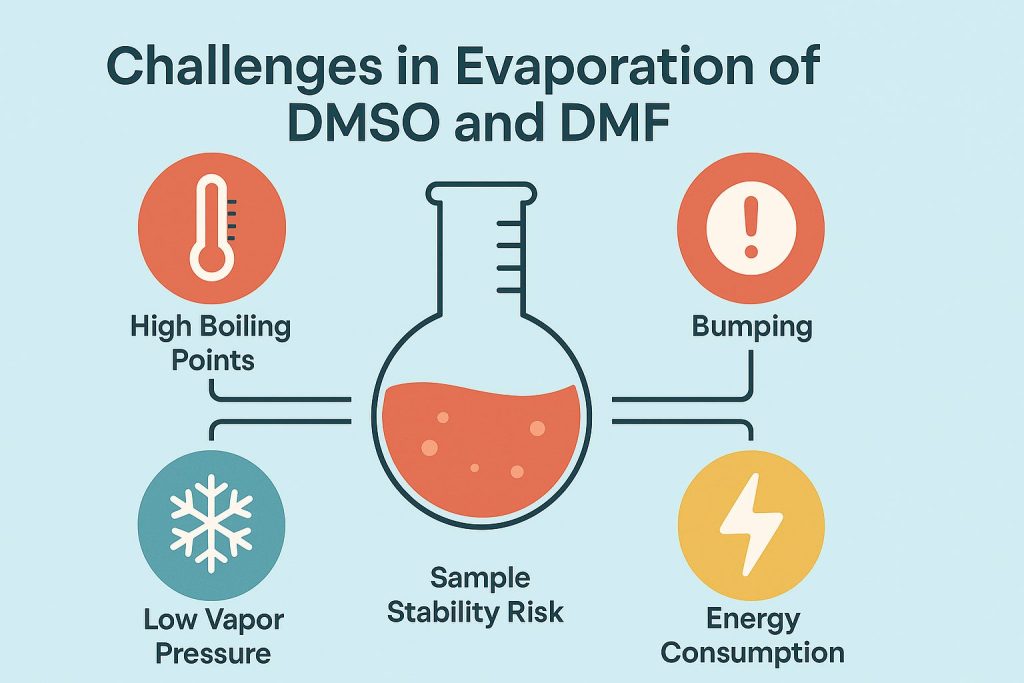
High Boiling Points Demand More Energy
DMSO boils at 189°C (372°F) and DMF at 153°C (307°F)—far above water. To evaporate them, rotary evaporators must use:
Hot water baths at 70–90°C
Strong vacuum conditions (often below 10 mbar)
This results in slow evaporation and high energy use, especially for small-volume samples in narrow containers.
Bumping: A Risk for Sample Loss
Another issue is bumping—violent, sudden boiling under vacuum that can eject your sample.
Even with:
Slow pressure reduction
Anti-bumping granules
…bumping is hard to fully control, making workflows less predictable.
Risk of Sample Degradation
High heat and vacuum can degrade sensitive compounds, especially:
Bioactive molecules
Precision-targeted samples
This compromises result quality in experiments requiring high accuracy.
Physical Properties Make It Worse
DMSO and DMF have high specific heat capacities and low vapor pressures, meaning they require more thermal energy to evaporate and still do so slowly. Their strong solvent properties make them cling to samples even after long evaporation times, making it hard to weigh the final sample or proceed to the next analytical step.
What an Ideal Evaporation System Needs
To address these issues, a better solvent evaporation system should:
✅ Provide stable, gentle heating at low temperatures
✅ Operate under safe vacuum or atmospheric conditions
✅ Prevent bumping without additives
✅ Handle small volumes efficiently
Recognizing these difficulties is key to choosing better tools and improving efficiency in solvent evaporation.
📋 Challenges of Evaporating DMSO and DMF
| Challenge | Why It Happens | Impact on Workflow |
|---|---|---|
| High boiling points | DMSO: 189°C (372°F), DMF: 153°C (307°F) | Requires high heat and deep vacuum |
| Bumping | Sudden boiling due to unstable vacuum conditions | Risk of sample loss or contamination |
| Thermal degradation of compounds | Heat- or vacuum-sensitive compounds may break down | Unreliable data, especially in sensitive experiments |
| Low vapor pressure and high heat capacity | Slow phase change even under optimal conditions | Long evaporation times |
| Strong solvent retention | Solvents cling to samples and remain even after evaporation | Difficult weighing and analysis of dry samples |
Ready to Reduce Energy Use Without Compromising Research?
3. How Smart Evaporator™ Reduces Energy and Time
A Revolutionary Alternative to Conventional Methods
Smart Evaporator™ offers a fundamentally different approach to evaporating solvents like DMSO and DMF, which are notoriously hard to remove due to their high boiling points and low volatility.
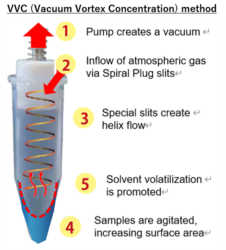
At the heart of this innovation is Vacuum Vortex Concentration (VVC) method. Unlike traditional systems that rely on deep vacuum and high-temperature water baths, VVC uses spiraling airflow to agitate the sample, enhancing evaporation without thermal stress—all at atmospheric pressure.
Reduces Bumping and Energy Use
This method also:
Prevents bumping, a common issue in vacuum-based systems
Protects thermally sensitive compounds from degradation
Requires no water bath, chiller, or large vacuum pump
Uses significantly less energy than rotary evaporators
Enables unattended operation, ideal for multi-tasking labs
Proven Benefits in Energy and Workflow
For laboratories frequently working with stubborn solvents like DMSO and DMF, the Smart Evaporator™ represents a practical solution that balances efficiency, sample safety, and sustainability. Smart Evaporator™ uses up to 90% less energy compared to traditional rotary evaporators. (According to BioChromato’s proprietary research)*
📊 Feature Comparison at a Glance
| Feature | Smart Evaporator™ Approach | Benefit |
|---|---|---|
| Evaporation Method | VVC at atmospheric pressure | No need for deep vacuum or water bath |
| Sample Agitation | Spiral airflow increases surface area | Faster evaporation, no thermal stress |
| Risk of Bumping | Gentle atmospheric-pressure operation | Reduced bumping risk |
| Thermal Sensitivity | No extended high heat exposure | Preserves bioactive/sensitive compounds |
| Equipment Requirements | No chiller, water bath, or large vacuum pump | Lower energy usage, simplified setup |
| Energy Consumption | Minimal power input | Up to 90% energy reduction* |
| Lab Efficiency | Unattended operation possible | Saves time, improves workflow |
| Sample Recovery | Effective even with delicate or small samples | Higher yield, better reproducibility |
Discover the Smart Evaporator™: The Safer, Simpler Solution for DMSO and DMF Removal
4. Smart Evaporator™ in Action: Trusted by Leading Institutions
Top universities and research labs around the world are turning to Smart Evaporator™ for its unique combination of efficiency, simplicity, and reliability—especially when dealing with difficult solvents like DMSO and DMF.
Real-World Lab Experiences
University of California, San Diego (UCSD)
At UCSD, researchers were able to recover sensitive compounds without constant supervision, making their workflows more efficient and less labor-intensive.
Korea University
The Smart Evaporator™ enabled faster evaporation of water and DMSO, significantly streamlining NMR sample preparation.
Purdue University
Dr. Davisson highlighted that efficient DMSO removal at a practical scale and speed remains a major unmet need in pharmaceutical research. The Smart Evaporator™ addressed this challenge effectively.
The gentle evaporation process also improved sample recovery, which is critical when working with precious or limited compounds.
➡️ See more real-world feedback from researchers: Smart Evaporator™ User Voices
Discover the DMSO Evaporator™: The Safer, Simpler Solution for Solvent Removal
5. Summary
Handling high-boiling solvents like DMSO and DMF presents unique challenges: extended evaporation time, high energy consumption, and risks of compound degradation. Traditional tools such as rotary evaporators often require deep vacuum and long runtime to overcome these issues.
Smart Evaporator™ provides a smarter alternative—achieving gentle evaporation at atmospheric pressure while minimizing energy use. By eliminating the need for chillers, large vacuum pumps, or water baths, it offers labs a way to improve throughput, reduce power demand, and better preserve sample integrity.
Ready to Reduce Energy Use Without Compromising Research?
Explore energy-efficient solutions designed to meet your lab’s unique needs.
📚 Related Articles
- Rotary Evaporator vs. Energy-Efficient Alternatives: How to Cut Evaporation Time and Power Usage👉 Read the full article here
- Is Your Lab Equipment Wasting Energy? Breaking Down the Hidden Power Costs in Research Labs👉 Read the full article here
- Rotavap and Smart Evaporator: 2 Use Cases and Why It Works👉 Read the full article here
- Maximizing Lab Efficiency: How Smart Evaporators Complement the Rotary Standard👉 Read the full article here
- Latest Trends in Evaporation Technology: What Equipment Will Laboratories Need in the Future?👉 Read the full article here
Sources
- U.S. Department of Energy – Energy Efficiency in Laboratories