Improvement of fraction recovery rate from alcohol-containing sample
~Japanese Sake~
【Keyword】 Japanese sake ethanol recovery rate improvement fractionation Conveni-Prep
Introduction

- As it is said that “sake is the chief of medicine” since ancient times, Japanese sake contains many active ingredients.
- Solid phase extraction is often used for discovery work for active ingredients in Japanese sake. However, when Japanese sake is used as it is as a sample without pretreatment, there is a problem that the yield at fractionation decreases by the influence of alcohol.
- It is possible to improve the yield of fractionation from the sample containing alcohol by using ” Conveni-Prep” , which is a fractionation system utilizes the patented VVC method* for suction agitating at the sample application.
*Vacuum Vortex Concentration : VVC, Fig.2
Mechanism of suction vaporization solid phase extraction method
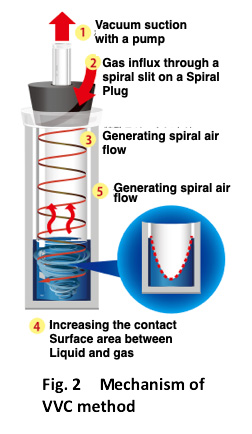
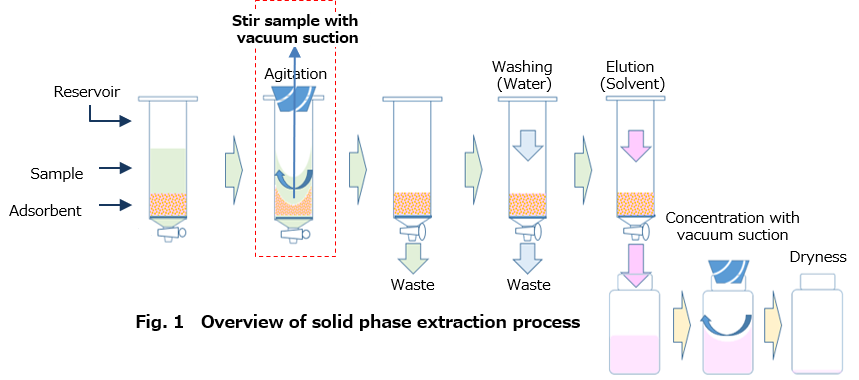
- This device is a fractionation system using the patented VVC method, and it is possible to concentrate and dry the fractionated sample as it is in one device.
- The flow of each step of the fractionation work is shown in Fig.1. When the sample is placed in
the reservoir filled with the adsorbent, "suction agitating" is performed by the VVC method to
promote vaporization of the alcohol content in the sample, resulting in reduction of alcohol concentration (Fig. 1 red line part). - Since the adsorption amount which has been influenced by the alcohol content can be increased , the fractionation recovery rate can be improved.
Sample
・Japanese Sake (Dai-ginjo quality, Alcohol content:15~16%)
Experiment
<Basic operation>
- Add 10 g of aromatic synthetic adsorbent to the reservoir and perform conditioning with methanol.
- After adding 20 mL of sample to the reservoir, perform "suction agitation" for a certain period of time (suction amount: 25 L / min).
- Discharge the sample from reservoir.
- Add 20 mL of water to the reservoir and wash the adsorbent.
- Add 15 mL of methanol to the reservoir and receive the solvent extraction fraction in a vial.
- Concentrate and dry solidification by vortex and suction while maintaining the extract solution in the vial at 50 ° C.

<Evaluations>
① Influence of "suction agitating time" in recovery rate
Confirmed the amount of solvent extraction fraction when the suction agitating time is varied from 0 to 90 min.
② Evaluation of solvent extraction fractionation components
The solvent extraction fractions obtained at two different suction agitating times "none" or "90 minutes“ are analyzed
by thermal desorption / pyrolysis DART®-MS and are compared.
Results
① Influence of "suction agitating time" in recovery rate
Tab.1 and Fig.4 show the amount of the dried and solidated fractions finally obtained by the solvent extraction. The time of "suction
agitating", which is carried out when the sample is added to the solid phase (adsorbent), is varied. As a result, the amount of fractions
increased by "suction agitating“; the yield increased about twice at 90 min. With the use of “Conveni-Prep” , "suction agitating “ can be
carried out while the solid phase extraction process. It easily makes the improvement of recovery rate for the solvent extracted fractions
from the alcohol containing samples.

② Evaluation of solvent extraction fractionation components
The obtained solvent extracted fraction was analyzed by thermal desorption / pyrolysis DART®-MS . There is no apparent deference
between the components obtained with or without “suction agitating”(MS spectrum in the Fig.6 upper) . However, when compared by
the chromatogram (EIC) of individual peaks, there is a component whose extraction amount does not change (eg: m/z 226) or
components whose extraction amount increase (for example, m/z 272, 434).

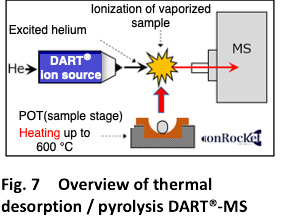
(Supplement)Thermal desorption / pyrolysis DART®-MS
The sample is vaporized by heating the sample linearly from room temperature to 600°C. Since the components in the sample are directly ionized for mass spectrometry, simultaneous analysis can be performed without treatment even for samples having complicated compositions. Fig.7shows the system outline. It is also a feature of the system that it has quantitative ability.
Conclusion
In addition to alcoholic beverages, this method will be useful for increasing of the fractionation yield for alcohol extracts from food samples, culture media, etc. (Increase in yield of precious components, simplifying pretreatment ).
