Differences in Aromatic Components of Cola
Purpose
Cola is a carbonated beverage blended with multiple flavors and is a popular drink sold by various manufacturers. Additionally, homemade craft cola recipes that combine various spices and herbs are shared online. This time, we conducted an experiment to determine whether differences can be identified between two types of commercially available cola using ionization by ChemZo and differential analysis (IDS) with Spectra Scope.

Methods
| Ion source | ChemZo (BioChromato) |
| Mass Spectrometer | compact QTOF (Bruker) |
| Measurement Method | Two types of commercially available cola, A and B, were introduced into the mass spectrometer (MS) through a tube to analyze their aromas. The samples were measured at room temperature in positive mode, with each sample measured for 30 seconds, repeated three times. |
| Data Processing | The aromatic components that differed between cola A and B were extracted using Spectra Scope's differential analysis (IDS). Subsequently, a database search was performed with Compound Search, and the estimated molecular formulas were listed. |
Results
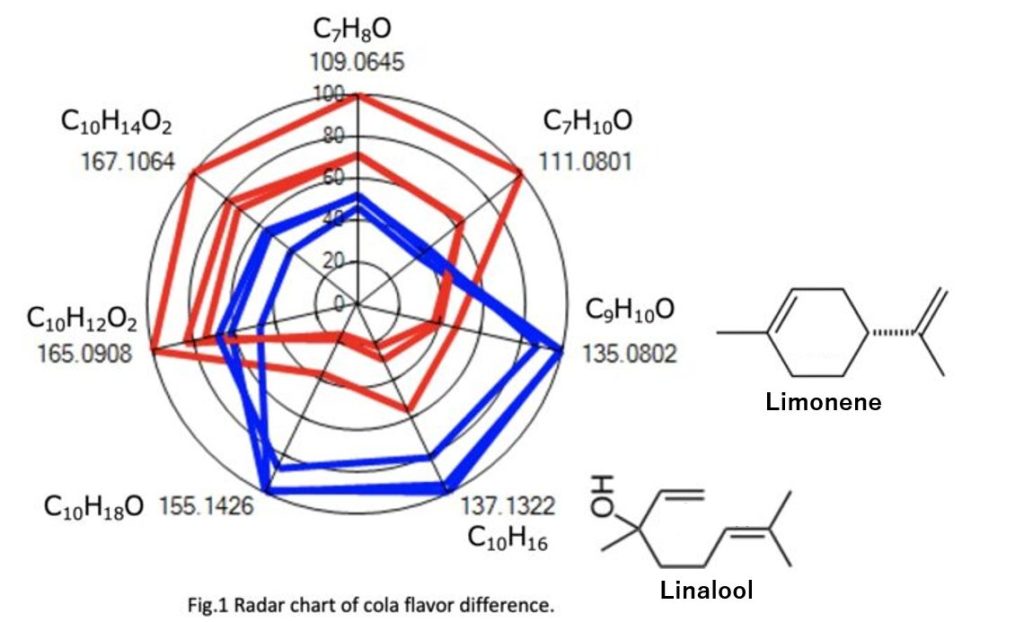
The components that differed between the two colas were automatically extracted using IDS, and the results were presented in a radar chart (Fig. 1). Multiple aromatic components showed intensity differences between cola A (red) and cola B (blue). For example, m/z = 135.0802, m/z = 137.1322, and m/z = 155.1426 were detected in both colas, but their intensities were more than twice as high in cola B compared to cola A.
Colas are known to use citrus flavors. Based on the measurement results of standard substances, m/z = 135.0802 is estimated to be the dehydrogenated peak of limonene, while m/z = 137.1322 is estimated to be the dehydration peak of linalool.