Useful for adjusting the solubility of luminous rare earth complex and for removing the solvents!
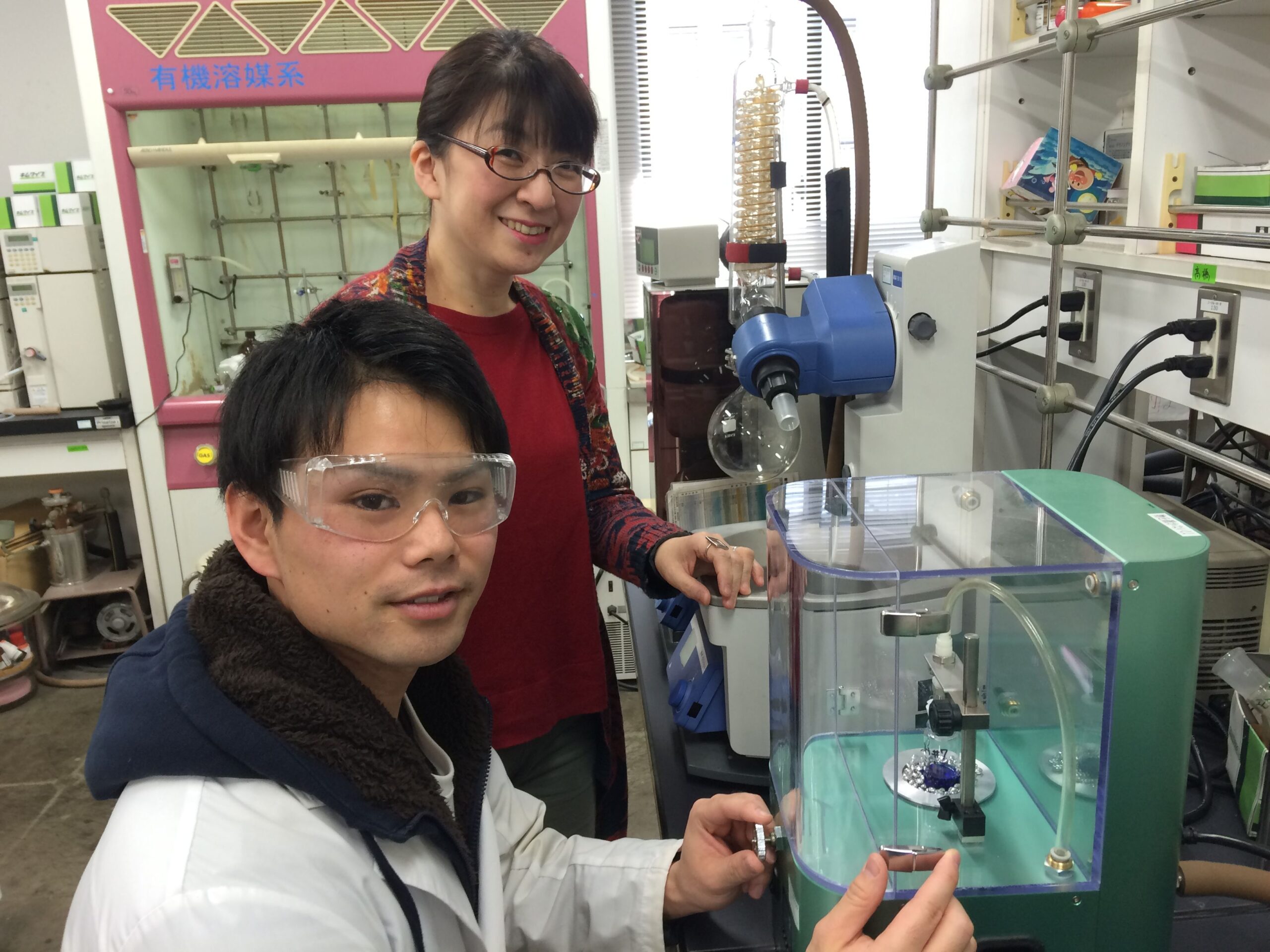
This time I talked to Ms. Hasegawa who is developing the luminous rare earth complex and working on clarifying its mechanism.
Interviewee: Prof. Miki Hasegawa, Photo functionality of Coordination Complexes, College of Science and Engineering, Aoyoma Gakuin University
BioChromato
Thank you for your time today. What type of research are you in charge of?
Prof. Hasegawa
We are developing the luminous rare earth complex that reacts to the environment, and working on clarifying the mechanism. One of the familiar applications is for the fluorescent ink used to for prevention of banknotes forgery, but it’s been said that only a part of material’s potential ability is being used. We aim to understand the structure of the rare earth through the basic research.
BioChromato
I see. How do you use the Smart Evaporator?
Prof. Hasegawa
I use that for adjusting the solubility of rare earth complex that has the high solubility to the solvent and also use for removing the solvents.
BioChromato
What is the process before and after the evaporation?
Prof. Hasegawa
First we synthesis the sample at small volume, evaporate the solvent, and then carry out the element analysis or NMR to understand the structure.
BioChromato
What is the evaporation condition?
Prof. Hasegawa
We put the 2ml of water or organic solvent like Acetonitrile or ethanol in the 10ml screw bottle, and brig those to complete dry. The heather is set at 40Celsius to 60Celsius. It’s operated for 3-4 hours a day by about 10 students almost every day, and each evaporation takes 20 minutes.
BioChromato
Appreciated to know that it’s been used constantly. Do you also use the other types of evaporators?
Prof. Hasegawa
I use the rotary evaporator for evaporating the half of large sample, or for making samples to thin layer. Sometimes I use the desiccator too. I used to use the Kugelrohr before, but now I changed to the Smart Evaporator.
BioChromato
Now you are using the different apparatus, and how was your process changed?
Prof. Hasegawa
The number of operations is reduced, and it’s now easy to handle small sample till the synthesis. Until now, after removing solvent with the 300 ml or 500ml flask-type rotary evaporator, the sample was transferred to a screw tube and dried with a desiccator. Now I don’t have to transfer for many times, and efficiency has been much improved by having less sample loss. When working with a small amount of solvent, I always worked carefully.
BioChromato
Now you can do the evaporation with the screw tube where the sample is kept for storage afterwards, and is it the biggest factor improving your efficiency?
Prof. Hasegawa
Right. No more transferring and no time-consuming so I can readily proceed to the structure grasp. The Smart Evaporator works just fine especially for the element analysis.

BioChromato
Why is it good for the element analysis?
Prof. Hasegawa
Because the system does not damage the sample, but still is able to bring to the complete dry. The 2ml of solvent is large enough for analysis, but when it does not dry up completely, that starts joining up with the other component which makes the totally different substance. This is what we call “quenching phenomenon”, and we have the different light function and intensity. I thank to the Smart Evaporator that has the transparent cover, which makes the sample process visible to keep eyes until the complete dry.
BioChromato
So there’s low risk to have quenching phenomenon now, and you can readily proceed to the analysis. Do you have any other changes by using the Smart Evaporator?
Prof. Hasegawa
I feel that the number of failures has reduced and the operation safety has also improved.
For example, when using the Kugelrohr to remove impurities, the solid sample sometimes gets burdened, or the sample bumps, we breaks the glass..etc. It was a work under tension. Now there is no bumping risk and no breakage risk, so everyone can work readily. If used with the oil bath that often contaminates the bottle surface, but with the Smart Evaporator the aluminum beads should not. I also thank to this.
BioChromato
The sample loss by failures is reduced, the number of operations is reduced, then those result in more efficient experiments.
Prof. Hasegawa
The rare earth complex is a field with many unknown elements, but I would like to pursue the possibility of chemistry development by clarifying the mechanism. We need to keep our research highly motivated, and we feel the Smart Evaporator is a valuable equipment which let us easily work with confidence.
BioChromato
We are honored to hear that. Thank you very much.
Summary
Professor Hasegawa is conducting fundamental research to elucidate the mechanism of the luminescent rare earth complex, but she had an issue of the recovery rate of ingredients when conducting elemental analysis, X-ray diffraction, NMR. She used to work on evaporating solvent with 300 ml or 500ml of rotary evaporator flask, but there was a risk of sample loss and bumping when transferring from the flask to the screw tube, so she had to work with care. With the Smart Evaporator which can remove solvent with the screw tube, it reduces sample loss, bumping risk, and operations get shortened. Those contribute to increase her working efficiency. This time I realized again that the system letting people dry solvent without transferring samples increases the sample recovery ratio and is effective for elemental analysis and other relevant process.
(Interviewer: Kikuchi)
■Interested in what Smart Evaporator is? You can learn from here!
